In the last blog on Radioactivity, we learnt that some elements in nature emits radiation spontaneously. That is called Natural Radioactivity.
In 1934, husband-wife team of Irene Joliot and Frederic Joliot Curie were awarded Nobel Prize in Chemistry in recognition of their synthesis of new Radioactive elements.
The story behind discovery
Irene was the daughter of Marie and Pierre Curie and thus had large supply of polonium in her lab. Irene and Frederic did experiments with that. The polonium emitted alpha particles which they used to bombard on different.
One day they noticed when alpha particles where bombarded on aluminium sheet, it continued to emit Radiation (with half-life of approx. 3 minutes) even after the source was removed.
Playing with Nucleus – Be Careful
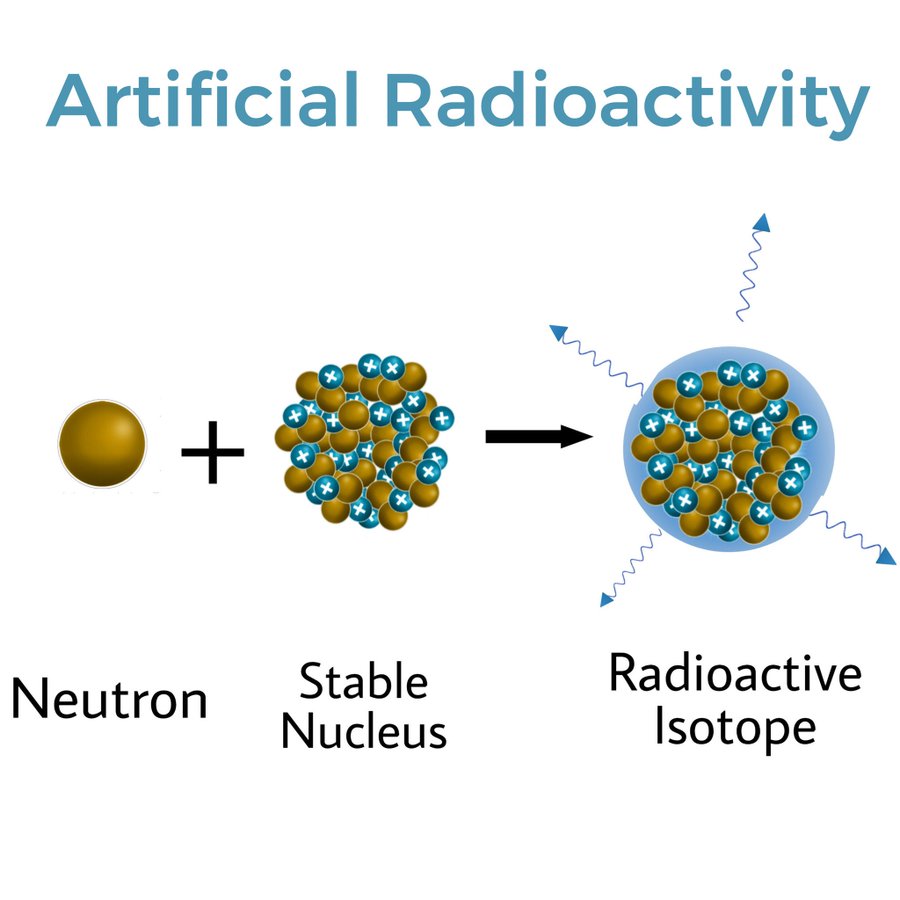
When alpha particle were bombarded on Aluminium Sheet, it interacted with the aluminium nucleus, resulting in nuclear reaction. The alpha particle is other name of the helium nucleus which is made up of 2 protons and 2 neutrons. This Alpha particle converted Aluminium into phosphorus-30 by emitting a neutron. Phosphorus-32 is stable but this new isotope made P-30 is radioactive element. This was responsible for radiation coming out of nucleus.
Irene and Frederic bombarded alpha particles on many other elements and found that it was possible to convert one element into other, with higher no. of protons in its nucleus. This is called Transmutation.
Thus this shows we are able to make isotopes artificially that are Radioactive.
Recipe of artificial radioactive isotope
There are many ways of making radioactive isotopes. The Basic idea is to make energy interact with the nucleus. Radioactive isotope can be made by bombarding an element with a particle like alpha particle, electron, neutron and high energy X rays.
Time to make New Equipment
By mid- 1930s several laboratories have equipment, such as accelerator called cyclotron to bombard protons, deuterons and alpha particles to form new radioactive isotopes.
But it was found that these particles are not that efficient. As these particles are positively charged it is difficult for them to interact with positively charged nucleus as they feel repulsion, specially while interacting with atoms with atomic number more than 20.
So what next?
Neutrons Helped!
A group of intelligent scientists, headed by Ernico fermi used neutrons to bombard on elements and found that neutrons are pretty efficient; as they have no charge and thus do not interact with the electromagnetic field of the nucleus.
Also, it was found that slow neutrons are more efficient to start nuclear reaction than fast neutrons.
The number of Artificial isotopes increased greatly after 1934.
This Breakthrough by Irene and Frederic curie led to the discovery and research on Nuclear fission.
Nuclear fission is possible!
This research resulted in the conclusion that Uranium can be split in two large fragments when bombarded with neutrons.
Nuclear fission: It is a process whereby energy is released by the splitting of Uranium Atoms. Fission releases heat energy that can generate steam, which is used to spin a turbine to produce electricity.
Nuclear Energy is considered to be renewable source and can produce enormous amount of energy.
It has more application then you would Expect.
These artificial radioactivity is used widely in medical field.
- Radiotracers : These are articicial radioactive isotopes that are injected into the patient’s body. It helps in the tracking of status of different body parts it passes through by tracking the movement of the radiation emitted by radioactive element.
- Radiotherapy : radioactive isotopes are attracted to tumors and stick to them as Radioactive elements can be easily detected in X-ray film we can easily know the damaged part and a whole map of it with the help of Radioactive Radiation.
Activation Analysis:
This is one my favorite applications of Radioactive Isotopes. This method can help to identify element in the the sample without destroying it.
Here’s How.
When a compound is irradiated with neutrons, many elements are activated and become radioactive. The radioactivity can be measured easily and the properties of the radiation can be used to identify an element. Thus, it is possible to observe with excellent sensitivity about 70 elements.
Rock from the Moon
It is also of interest to mention that when the composition of the moon was determined, the analysis was assisted by neutron activation. Rocks, brought back to Earth by the astronauts, were bombarded by neutrons, forming radioactive products. The subsequent radioactive emissions were then used to identify elements in the moon rocks.
There are many other Applications of artificial radioactive isotopes which are beyond the scope of this blog to cover.
Tell me in comment section below which is your favorite Application/use?